
This book is also part of a 3 book set known as the Bethlehem Science Book Set. There is a list of elements in the abbreviated form, and you have to identify the correct answer. Read the following questions carefully and answer accordingly. This book is suggested as a supplement to Seton's Science 7 for Young Catholics This quiz has been developed as fill in the blank format and Multiple Choice Type, which will test and improve your knowledge about elements name and their symbols. The first part of Wiker's witty and solidly instructive presentation is most suitable to middle school age, while the later chapters are designed for ages 12-13 and up, with a final chapter somewhat more advanced. He introduces the young reader to people like Von Helmont, Boyle, Stahl, Priestly, Cavendish, Lavoisier, and many others, all incredibly diverse in personality and approach, who have laid the groundwork for a search that is still unfolding to this day. Of course the wanderings made it an adventure, and an adventure is always an exciting thing to retell." Īuthor Benjamin Wiker leads the reader on a delightful and absorbing journey through the ages, on the trail of the elements of the Periodic Table as we know them today. It was a long and difficult journey much like the perilous wanderings of Odysseus in Homer's great epic tale, The Odyssey. This fact has key implications for the building up of the periodic table of elements."When we look at these nice, neat, and straight rows of elements on the Periodic Table of the Elements we might think that it was a nice, neat, and straight road to their discovery. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. As you move up and to the right on the periodic table: atomic radius increases and electronegativity increases. Otherwise, electron affinity increases moving left to right in a period and decreases moving down a group. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. In a period, the halogen will have the highest electron affinity and the noble gas the lowest. The total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol Z. In the periodic table, the elements are listed in order of increasing atomic number Z. I have the basic structure of the table (Using mostly div elements) but Im majorly lacking a responsive flow.
The periodic table chemistry test wizar code#
The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Lately, Ive been studying a lot about responsive design in HTML/CSS and decided to try to code the periodic table of elements in HTML/CSS in order to practice. The configuration of these electrons follows from the principles of quantum mechanics. Carbon and silicon are the basis of biological life and synthetic computing, respectively. Both lithium and sodium have low atomic weights.

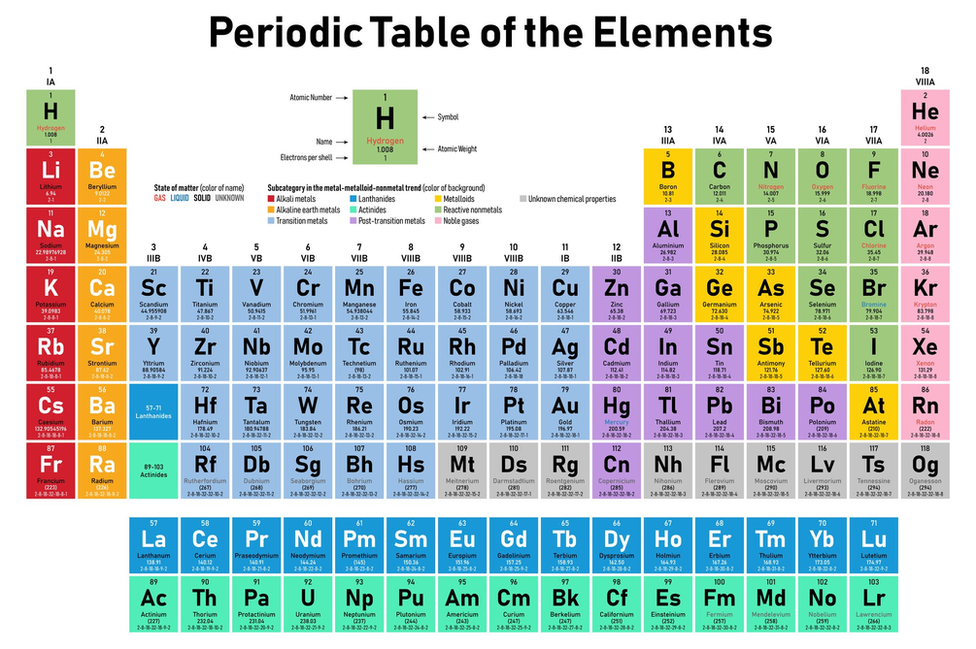
Lithium and sodium are in the same period of the Periodic Table. Lithium and sodium are in the same group of the Periodic Table. The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. Both lithium and sodium ions are positively charged. There is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. Generally, within one row (period) the elements are metals to the left, and non-metals to the right, with the elements having similar chemical behaviours placed in the same column.Įvery solid, liquid, gas, and plasma is composed of neutral or ionized atoms. It is organized in order of increasing atomic number. The periodic table is a tabular arrangement of the chemical elements.


 0 kommentar(er)
0 kommentar(er)
